Ocean Acidification
Ocean acidification is the ongoing decrease in the Earth's ocean pH, primarily caused by the absorption of excess carbon dioxide (CO2) from the atmosphere, leading to a more acidic ocean environment.
The term pH stands for potential or power of hydrogen with the “p” meaning potential or power and the “H” standing for hydrogen. The pH scale is a common and worldwide scale that is used to rank how basic or acidic a solution may be based on the amount of hydrogen ion activity in the solution.
The pH scale usually measures a substance from 1 to 14. The scale, however, is logarithmic. Each whole pH value below 7 (the pH of pure water) is ten times more acidic than the higher value and each whole pH value above 7 is ten times less acidic than the one below it. For example, a pH of 3 is ten times more acidic than a pH of 4 and 100 times (10 times 10) more acidic than a pH value of 5. A pH near 7 is considered to be neutral.
As concentrations of atmospheric carbon dioxide (CO2) increase, a majority of this CO2 is being absorbed by the ocean, as over 70 percent of Plant Earth is blue and made up of water. This triggers a series of chemical reactions that ultimately will lead to lower pH in seawater.
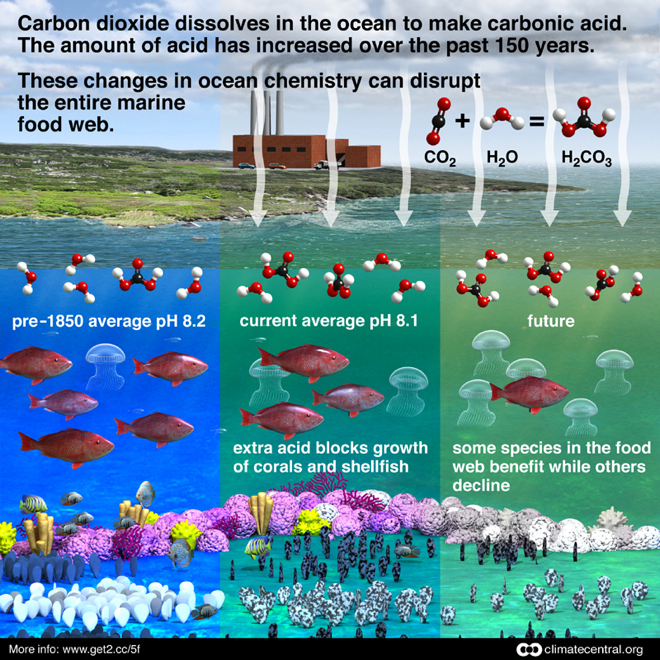
Since the Industrial Revolution, the average pH of surface ocean waters has decreased by 0.11 units, from 8.21 to 8.10. This may not seem significant, but since the pH scale is logarithmic, the 0.1 drop in pH corresponds to a 30% increase in ocean acidity.
When carbon dioxide (CO2) is absorbed from the atmosphere into the ocean, it reacts with seawater to form carbonic acid, making the ocean more acidic. The excess CO2 humans emit from the burning of fossil fuels and other activities not only leads to warming temperatures, it produces an ocean that is more acidic.
According to NOAA, since the beginning of the Industrial Revolution, the pH of surface ocean waters has fallen by 0.1 pH units. Since the pH scale, like the Richter scale, is logarithmic, this change represents approximately a 30 percent increase in acidity. Future predictions indicate that the oceans will continue to absorb carbon dioxide, further increasing ocean acidity. Estimates of future carbon dioxide levels, based on business as usual emission scenarios, indicate that by the end of this century the surface waters of the ocean could have acidity levels nearly 150 percent higher, resulting in a pH that the oceans haven’t experienced for more than 20 million years.
Ocean acidification is expected to impact ocean species to varying degrees. Photosynthetic algae and seagrasses may benefit from higher CO2 conditions in the ocean, as they require CO2 to live just like plants on land. On the other hand, studies have shown that lower environmental calcium carbonate saturation can have a dramatic effect on some calcifying or exoskeleton species, including oysters, clams, sea urchins, shallow water corals, deep sea corals, and calcareous plankton.
Just like humans, marine organisms require optimal conditions inside their bodies to stay healthy. If the acidity of seawater is beyond the optimum range for that organism, its body must use more energy to maintain healthy body fluid chemistry. Organisms can often compensate when faced with increased acidity, but this comes at the expense of using energy to grow critical body parts like muscle or shell.
For example, scientists have found that mussels, sea urchins, and crabs start to dissolve their protective shells to counter elevated acidity in their body fluids. Sea urchin and oyster larvae will not develop properly when acidity is increased. In another example, fish larvae lose their ability to smell and avoid predators. The vulnerability of larvae means that while organisms may be able to reproduce, their offspring may not reach adulthood.
THE BIOLOGICAL IMPACTS
Ocean Acidification reduces the size of clams
A consequence of increasing CO2 in the ocean is that it becomes harder for carbonate to mineralize in the water. What exactly does this mean?
Many organisms, including crabs, clams, mussels, and oysters, use calcium carbonate (CaCO3), a common substance found in rocks, to build their shells or outer-skeleton. A lower pH will make it more difficult for these organisms to produce a viable shell in the same shape and size as in the past.
In addition, calcifying plankton, which are at the base of a healthy aquatic food web, will also find it difficult or unable to form shells as a result of ocean acidification. This could have serious effects on species further up the food chain, including commercially valuable fish such as striped bass, blue fish, flounder, and cod.
Even a small reduction in pH makes it just a little bit harder for calcifying organisms to perform normal body functions. Organisms that use calcium carbonate to form shells are more likely to be negatively impacted by ocean acidification.
Ocean Acidification in Brief:
The Process:
When the ocean absorbs CO2 from the atmosphere, it reacts with seawater, forming carbonic acid (H2CO3) which then dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). The increase in hydrogen ions lowers the pH, making the water more acidic.
Causes:
The primary driver of ocean acidification is the burning of fossil fuels, which releases large amounts of CO2 into the atmosphere. The ocean absorbs roughly one-quarter of the CO2 released by human activities.
Impacts:
Shell Formation: Ocean acidification makes it harder for marine organisms, like corals, shellfish, and plankton, to build and maintain their shells and skeletons, which are made of calcium carbonate.
Ecosystem Changes: The changes in ocean chemistry can disrupt marine ecosystems, affecting the physiology and behavior of various species, potentially leading to shifts in the food web.
Specific Examples: Coral reefs, which are vital ecosystems, are particularly vulnerable to ocean acidification because their structure depends on the carbonate skeletons of corals.
Other Impacts: Ocean acidification can also affect the ability of some fish to detect predators and find suitable habitats. For example, the ability of some fish, like clownfish, to detect predators is decreased in more acidic waters. Studies have shown that decreased pH levels also affect the ability of larval clownfish to locate suitable habitat. When these organisms are at risk, the entire food web may also be at risk.
Current State:
The ocean's pH has already decreased by 0.1 units since pre-industrial times, representing a 30% increase in acidity.
Future Projections:
Scientists predict that the ocean's pH will continue to decline as atmospheric CO2 levels rise.
Global Issue:
Ocean acidification is a global problem, impacting every ocean on Earth, as well as coastal estuaries and other waterways.